48
LNG
INDUSTRY
OCTOBER
2016
Superficially, amine treating is simple. On the surface,
there are absorption and regeneration columns, with
solvent circulating between them in a closed loop through
a few pieces of heat transfer equipment, including a cross
exchanger and trim cooler, with a reboiler and overhead
condenser serving the stripper. However, beneath the
surface, the chemistry is complex. The system is reactive;
absorption and regeneration produce and consume large
amounts of heat; the solvent is a high strength ionic
solution, making the thermodynamics not ideal; and the
gas phase is often at extreme pressures of more than
100 bar. Modern treating frequently uses a solvent with
more than a single amine, which is almost always the case
in LNG production. It is extremely difficult to design such
facilities reliably without a clear understanding of the
physics and chemistry of the system as embodied, for
example, in a rigorous mass transfer rate-based simulator.
Given the high economic cost of the CO
2
removal section
of an LNG plant, it is surprising that antiquated simulators
are still used for design when the modern mass transfer
rate-based approach is commercially available. Using
dated tools results in needless design risk and a lack of
reliability, which can cause plants to only reach a fraction
of their nameplate capacity, or use oversized equipment.
Falling short
There are numerous reasons that LNG plants fail to meet
design throughput, either at start-up or after some time in
operation. The focus here is on the CO
2
removal section of
the LNG plant, and on some of the factors that can cause
design shortfalls and operating problems that limit plant
capacity. Apart from using an inadequate simulator and
unknowingly generating designs that are too tight, these
may include inadequate attention to the following in the
design phase:
Solvent contaminants, such as heat stable salts,
hydrate inhibitors (e.g. glycols, methanol), and amine
degradation products.
Changing blend composition because of disparate
amine volatilities.
Liquid and vapour distribution in towers.
Sensitivity to variation in parameters, such as changing
gas and liquid composition and coolant temperatures,
all of which can be determined by adequate sensitivity
studies.
Due to the presence of high concentrations of acid
gases (and to some extent degradation products), amine
systems are highly corrosive. This can lead to the
following:
Corroded or missing trays and corroded packing.
Leaking heat exchangers.
Plugged instrumentation and equipment from corrosion
products.
On the operations side, plants rarely run at steady
state. In attempting to win a construction bid, if a
contractor has developed a tight design without solid
knowledge of how tower internals actually perform from a
mass transfer (vs hydraulic) standpoint, normal process
fluctuations can throw a plant in and out of compliance
with meeting gas treating objectives. This list is by no
means exhaustive, but it does contain the majority of the
common causes for performance shortfalls. These will be
discussed individually in this article.
Tight designs
The expectation that the separation or gas purity
achievable by a given volume of packing should depend
on the packing type and size ought to be no surprise.
Therefore, gas treating process simulators should have a
rigorous way to account for the particulars of the tower
internals on the separation. Unfortunately, the designer
does not have this information and, as such, should
not be relied upon to provide this knowledge. It is easy
to calculate the number of ideal stages for a specified
separation, but it is difficult to take the results of the
calculation into an actual volume of specific packing. This
has been discussed in detail in a paper presented at the
2016 Laurence Reid Gas Conditioning Conference,
1
where
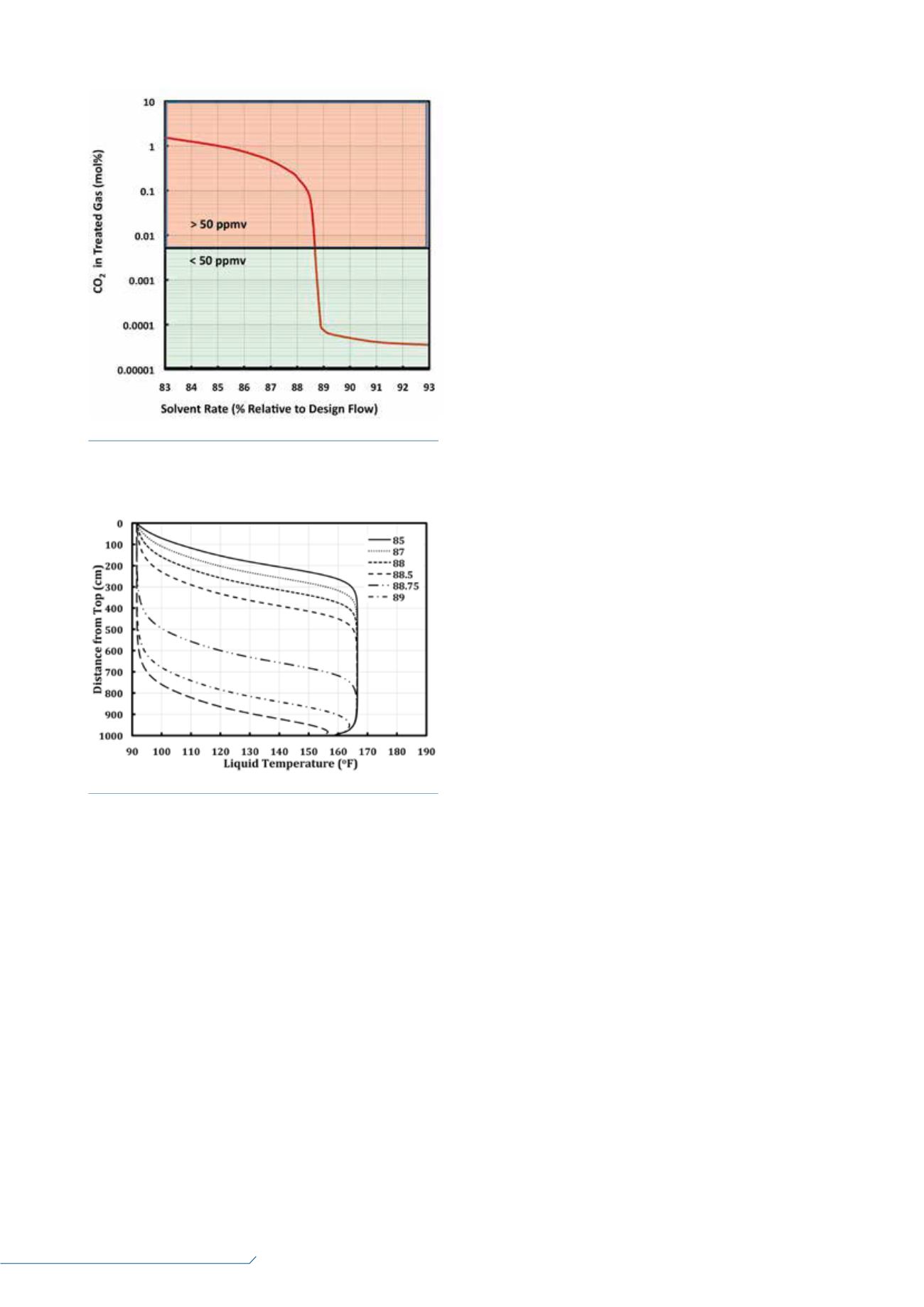
Figure 1.
CO
2
leak from a piperazine-methyldiethanolamine
(MDEA) absorber.
Figure 2.
Piperazine activated MDEA absorber temperature
profiles.


